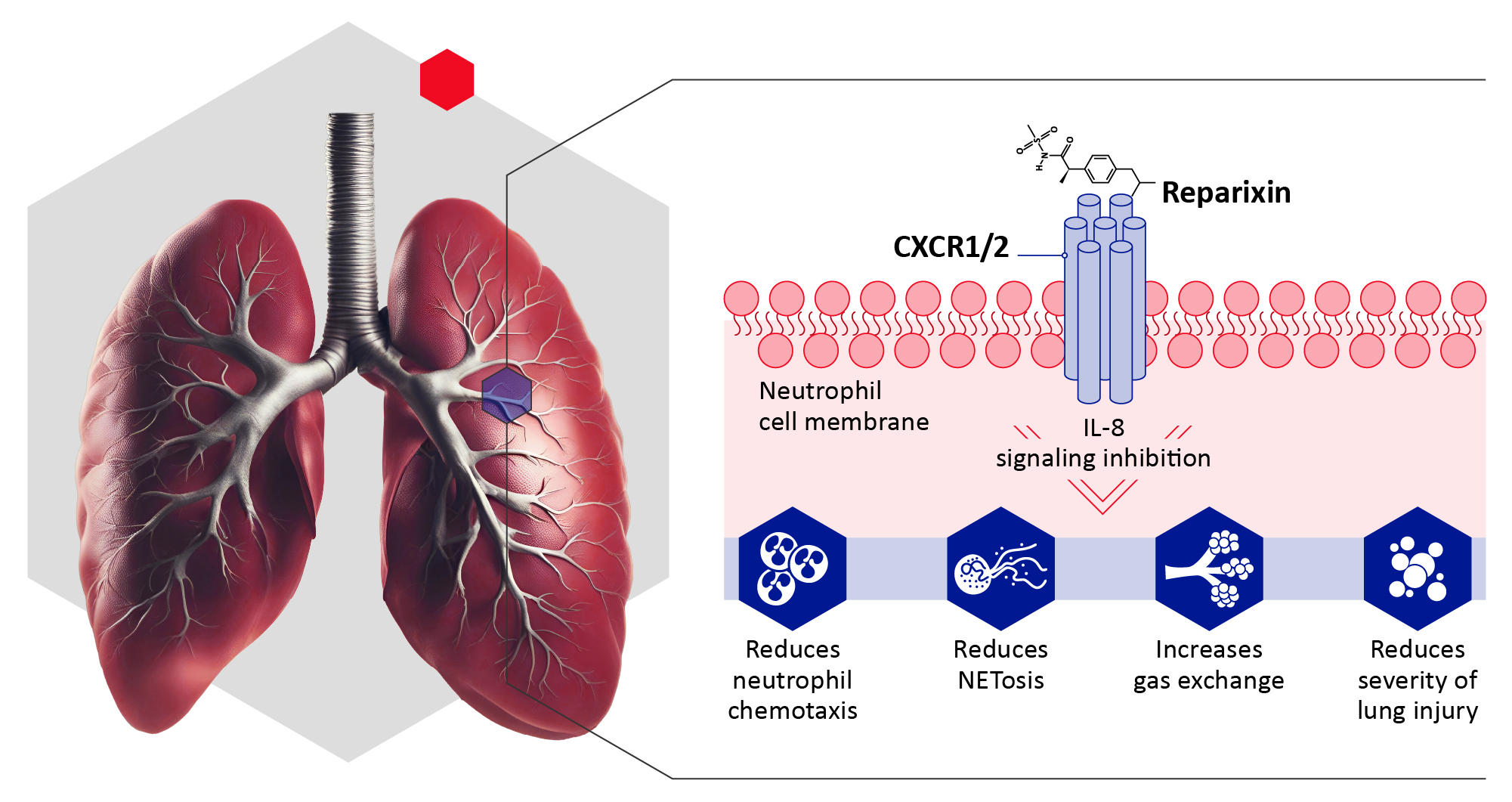
About the Investigational Drug:
Reparixin is an investigational, potent, noncompetitive allosteric inhibitor of the interleukin-8 (IL-8) receptors CXCR1 and CXCR2.1 Reducing interleukin-8 signaling may attenuate inflammatory responses by reducing neutrophil recruitment and neutrophil extracellular trap (NET) formation in severe community-acquired pneumonia, acute respiratory distress syndrome, and associated complications.2,3
During our completed phase 2 REPAVID-19 trial, patients with severe COVID-19 pneumonia who received reparixin exhibited a lower rate of clinical events, including need for supplemental oxygen, need for mechanical ventilation, admission to intensive care unit, or use of rescue medication compared to did those who received standard of care.4 In addition, patients with severe COVID-19 in a completed phase 3 trial showed less progression to the need for more invasive treatment than did those who received standard of care.5 Reparixin has been well tolerated in previous clinical trials of patients with breast cancer and COVID-19 pneumonia, with gastrointestinal discomfort being the most widely reported side effect.4,5