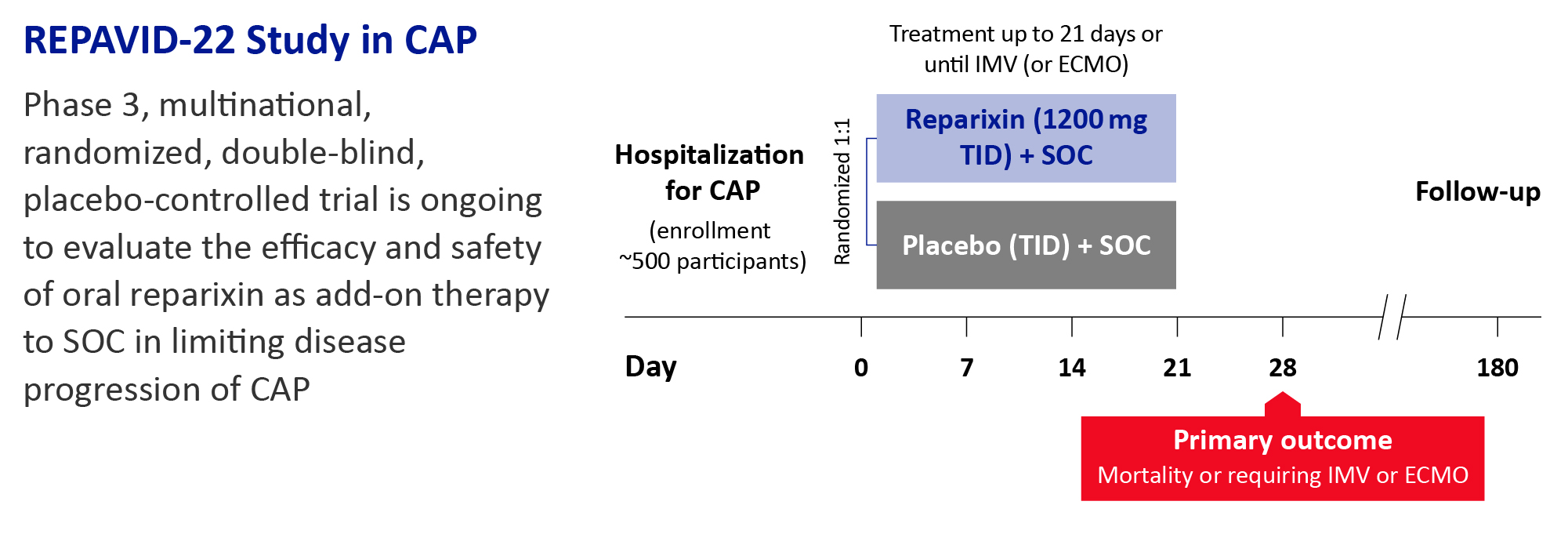
Treatment regimen
Participants will be randomized (1:1) to receive oral reparixin 1200 mg or placebo by mouth three times daily for up to 21 days and followed when they are discharged or if they are still hospitalized at Day 28 and up to Day 180. Participants will stop receiving reparixin or placebo before Day 21 if they are discharged from the hospital or receive invasive mechanical ventilation or extracorporeal membrane oxygenation. Antimicrobial treatment and supportive care will be provided according to clinical status and standard of care.
Target Population:
- Hospitalized adults (aged ≥18 years)
- Clinically suspected viral or bacterial CAP within 72 hours of hospital admission:
- ≥1 of the following signs/symptoms: Dyspnea, cough, purulent sputum, crackles (rales), or rhonchi
- Body temperature >38°C or <36°C (before or during admission) or leukocytosis (>local ULN)
- New/Increased pulmonary infiltrate(s) by chest imaging
- Need for noninvasive supplemental oxygen (not on mechanical ventilation)
- Not pregnant or planning to become pregnant
- No known hypersensitivity to ibuprofen or medications belonging to the sulfonamide class
- No active bleeding, previous intracranial hemorrhage, or recurrent peptic ulcer/gastrointestinal hemorrhage
- No current use of >2 immunosuppressive medications or immunosuppression status (i.e. systemic chemotherapy within the past 3 months, neutropenia, solid organ or bone marrow transplant recipients)
- No complex CAP-associated conditions such as fungal pulmonary infection, abscess, empyema, or pulmonary embolism
- Lack of renal or hepatic dysfunction
- Renal dysfunction: <50 mL/min/1.73 m2 eGFR
- Hepatic dysfunction: ALT or AST >5× ULN; Child-Pugh class B or C