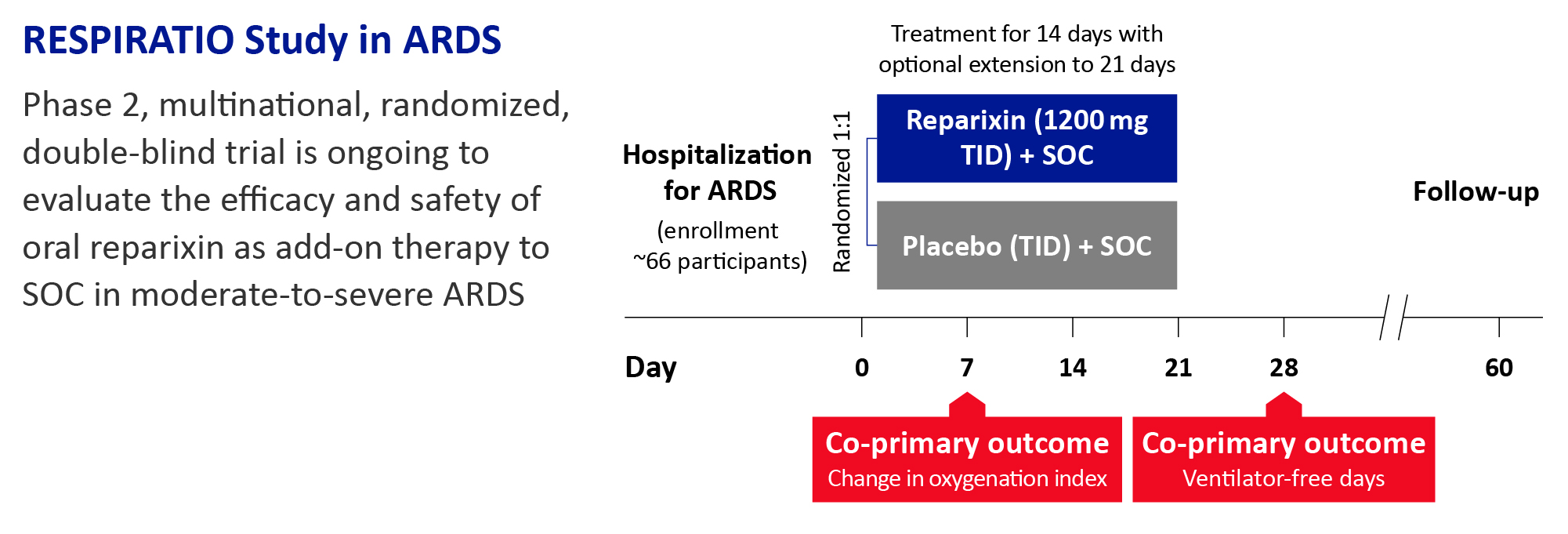
Treatment regimen
Participants will be randomized (1:1) to receive reparixin 1200 mg or placebo, in addition to standard of care, through a gastric tube three times daily for 14 days, with the option of extension up to 21 days if the patient is still intubated on Day 14. They will be followed until discharge, day 28, and up to day 60. ARDS treatment and supportive care will be provided according to clinical status and standard of care.
Target Population:
- Hospitalized adults (aged ≥18 years)
- Mechanically ventilated (invasive) patients with PaO2/FiO2 ratio ≤200 mmHg in the presence of PEEP ≥5 cm H2O
- Respiratory failure not fully explained by cardiac failure or fluid overload
- Within 48 hours from fulfilling ARDS diagnosis
- Not pregnant or planning to become pregnant within 30 days after the study ends
- No known hypersensitivity to sulfonamides, ibuprofen and other COX-1 and 2 inhibitors
- Not currently receiving ECMO or high-frequency oscillatory ventilation
- Lack of chronic renal or hepatic dysfunction
- Renal dysfunction:<30 mL/min/1.73 m2 eGFR or renal replacement therapy
- Hepatic dysfunction: AST/ALT ≥3× ULN + total bilirubin >2× ULN or AST/ALT ≥5× ULN; Child-Pugh Score 7 or higher